Batteries are available in different sizes, weights, voltages and capacities C, which refer to their stored energy expressed either in amps-hour Ah or milliamps-hour mAh. For example, a battery with a capacity of 500mAh should deliver 500mA during one hour before it gets totaly discharged (flat).
The NiMH have higher capacity/weight compared with the NiCads but are
The rate of self-discharge doubles for a rise in temperature of 10 degrees C. Some NiCads can discharge themselves completely in a period of six months.
The best way to keep batteries which are not in use for a long time, is by having them stored in the refrigerator (not in the freezer).
Just allow the battery to reach the ambient temperature before using/recharging.
Some manufacturers claim that these battery types are able to stand at least 1000 charges/discharges during their lifetime, assuming they have been subject to the ideal charging and handling methods.
In practice however, we may expect about 600 - 800 charges/discharges. A safe method to charge both the NiCads and the NiMHs is by using a constant charge current (CC) at 1/10 of their capacity (0.1C) during 14 hours. For other charge current values one may use the following formula:
Charge Time (Hours) = 1.4 x Battery Capacity / Charge Current (assuming that a constant charge current is used).
However, low cost CC chargers provide no way of detecting when the battery is fully charged. The user is then expected to estimate the charging time based on the constant charging current value and the battery capacity, according to the formula above. And providing the NiCads' are discharged to about 1.1V p/cell each time before recharging, this charging method can be used to achieve a reasonably long battery life. Since repeatedly recharging an already fully charged NiCad or one with a large part of its charge remaining will degrade its performance.
Some chargers provide the option to discharge the batteries down to about 1.1V per cell before starting the charging process.
There are also fast battery chargers on the market charging from 1C up to 4C. But due to the high charging current level, it is required a reliable method of stopping the charge once the battery is fully charged, otherwise overheating and battery damage may occur.
Since the NiMHs' and NiCads' voltage actually starts dropping after they have reached the fully charged state, the fast chargers use the so-called Delta Peak detecting method.
There are "negative delta V (-DV)" and "zero delta V (0D)" detectors. Also "change of temperature (dT/dt)" detectors are commonly used. Some manufacturers use negative or zero delta V together with change of temp. detection, in case of one method fails to detect.
Since NiMHs' voltage drop (delta V) after the fully charged state is lower than the NiCads, a more sensitive delta V charger is required for the NiMH batteries.
Some chargers allow the user to set the value of the delta peak detection, which may be between 10 - 20mV per cell for NiCads and 5 - 10mV for NiMHs.
A too low value may cause false peak detection due to electric noise, preventing the batteries from getting fully charged, whereas a too large value may result in overcharge, which reduces the batteries' life.
Some fast chargers offer the possibility to automatically change over to slow
charge (trickle-charge, for ex. at 0.05C) when the fully charge status is detected.
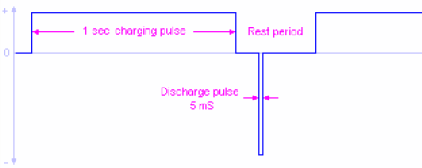
The graph on the right shows the voltage and temperature variation of a four cell NiCad during charging at 1C constant charge current.
Notice how the voltage drops after it has reached a top value, whereas the temperature keeps rising.
The battery is considered fully charged when the temp. rises about 10°C above the ambient temp. (e.g. 24 + 10 = 34°C )
It's not possible to know exactly the NiCad's or NiMH's cell charge status by only measuring it's terminal voltage, as the cell's charge status is not a linear function of the cell's voltage. A reliable method to know how much charge is left or whether a cell still has its nominal capacity, is by discharging it with a known constant current and measure the time until the cell voltage reaches about 1.1V. For example, it should take about two hours to discharge a fully charged 500mAh cell by using a constant discharging current of 250mAh.
This is particularly true at the higher charge rates used by fast chargers. These chargers have a microprocessor that samples the "rest" periods between the charging pulses to read the battery terminal voltage. Another interesting discovery is that the charging process actually improves even further if during the "rest period" between charging pulses, the cells are subject to very brief discharging pulses with an amplitude of about 2.5 times the charging current, but lasting only about 5mS.
It's claimed that individual cell differences may level out by slow charging the battery pack from time to time at 0.1C during 14h or so. For those who like to tinker with electronics and can't afford an expensive and sophisticated charger, there's a cheap alternative based on the National Semiconductor LM317 low cost regulator. The circuit diagram below shows a constant current charger using the LM317.

The constant current may be set anywhere between 10mA and 1.5A by choosing the appropriate resistor R.
R = 1.25 / I
Where R is the resistor value in ohms, 1.25 is a reference drop voltage in Volts and I is the constant current in Amps.
For example, to charge a 500mAH battery at 0.1C, (50mA) the R value will be:
1.25 / 0.05 = 25ohm.
The dissipated power on the resistor R in this example is:
P = V x I = 1.25 x 0.05 = 0.0625W or 62.5mW.
The dissipated power on the LM317 IC is:
(Vin - Vout) x Charging Current.
It's advisable to use a heatsink to prevent the IC from getting too hot. Notice that the IC's metal package or tab also carries the Vout, so it's necessary to use isolating washers in case you attach the heatsink to a metal case.
NiCads and NiMHs may be on charge during relatively long time without the risk of overcharging damage when using a constant current equal or less than 0.1C. However, it is not advisable to have the batteries continuously on charge longer than 24h, so one may connect the charger to a timer in order to cut the charging after about 14 -18h.
For those who prefer a more sophisticated D.I.Y. NiCad charger based on delta peak method, as well as other interesting circuits, check here
New rechargeable battery types, such as the Li-Ion (liquid electrolyte), the Lithium-Ion-Polymer (gel flat electrolyte) and especially the Lithium-Polymer (solid polymer electrolyte) are now often used with slow-flyers, indoors and even in much bigger models.
A Lithium-Polymer cell (Li-poly or Lipo) has 3.7V nominal voltage, 4.2V max and 3.0V minimum. Other types may have different nominal voltages. These battery types have much higher energy density than NiCads and NiMHs.
The max charge rate recommended is 1C, while the discharge rate should not be higher than 3 - 4C continuous or 5 - 6C during short time for the earlier types. Nowadays however some manufacturers offer discharge rates up to above 20C.For the same capacity, the battery with higher recommended max discharge rate has lower internal resistance, which provides better ability to deliver power. The self-discharge rate is claimed to be very low, typically 5% per year.
These batteries cannot be charged with the same chargers that are designed for only NiCads or NiMH.
In order to correctly charge the Li-ion/Lithium-polymer batteries, it must be taken into account the number of cells in the actual battery pack, since both the max charging current and voltage have to be set according to the cells' specifications.
Charging these batteries with a wrong charger may cause them to explode! Also a short circuited pack may easily catch fire. According to Kokam, the Lithium-polymer batteries should not be discharged below 2.5V per cell, otherwise a rapid deterioration will occur.
The basic charging procedure is by limiting the current (from 0.2 C to max 1C depending on manufacturer) until the battery reaches 4.2 V/cell and keeping this voltage until the charge current has dropped to 10% of the capacity C. Since the batteries only have 40 to 70% of full capacity when 4.2V/cell is reached, it's necessary to continue charging them until the current drops as described above. A charge timer should be used to terminate the charge in case the top voltage and/or termination current never reach their values within a certain time, which depends on the initial charging current, (e.g. 2 hours at 1C or 10 hours at 0.2C). Trickle charging is not good for Lithium batteries, as the chemistry cannot accept an overcharge without causing damage to the cells.
Panasonic's charge curve for their 830mAh cells is shown below:
![lipo_curve[6] lipo_curve[6]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEi8dR1-UDFttqlwsEkwhHMydvCj4e-vmTPfQHMshpvLsXBW6BnZ0S6ojjtF6vyT1aYDuSW_F7_031K4RPPL18hZr8FlZSNpe9C6i4BAO81GlW-fBIfT3QJ7FrWEBUQkJCVUKjLeHrTIUP4/?imgmax=800)
The circuit diagram below shows a simple Li-ion/Lithium-polymer charger based on National Semiconductor LM317 low cost regulator.

Before connecting the cells to the charger the max charging voltage has to be set by adjusting P1 (2k potentiometer). The max charging voltage must not exceed 4.2V per cell (Kokam), e.g. 8.4V for two serial connected cells. It is recommended using a digital voltmeter. The max charging current is set by choosing the value of Rx. Rx = 0.6 / max charging current
For example, for a max charging current of 600mA, Rx should be 0.6 / 0.6 = 1ohm, while for a max charging current of 1.2A it should be 0.6 / 1.2 = 0.5ohm. The dissipated power on Rx at a charging current of 1.2A is:
P = V x I = 0.6 x 1.2 = 0.72W
The dissipated power on the LM317 IC is:
(Vin - Vout) x Charging Current.
It's advisable to use a heatsink to prevent the IC from getting too hot. Notice that the IC's metal package or tab also carries the Vout, so it's necessary to use isolating washers in case you attach the heatsink to a metal case.
The LM317's max output current is 1.5A. For higher charging currents one may use the LM350 rated at 3A or the LM1084 rated at 5A.
Note: if a Li-ion battery gets discharged below 2.9V/cell, it needs to be slow charged at 0.1C until 3.0V/cell is reached before a higher charging current rate may be used. Also discharging below 2.3V/cell will damage the battery.
According to the manufacturers the Li-ion batteries should be stored charged to about 30 - 50% of capacity at room temperature. For prolonged storage periods, store discharged (i.e. 2.5 to 3.0V/cell) at -20° to 25° C.
Important!
Make sure to set your charger to the correct voltage according to the number of cells.
Failure to do this may result in battery fire!
Before you charge a new Lithium pack, check the voltage of each cell individually.
This is absolutely critical as an unbalanced pack may explode while charging even if the correct
cell count was chosen.
If the voltage difference between cells is greater than 0.1V, charge each cell individually to 4.2V
so that they are all equal.
If after discharge, the pack still is unbalanced you have a faulty cell that must be replaced.
Do not charge at more than 1C.
NEVER charge the batteries unattended.
Caution:
If you crash with Lithium cells there is a risk that they get a latent internal short-circuit.
The cells may still look just fine but, if you crash in any way remove the battery pack carefully
from the model and place it on a non-flammable place, as these cells may catch fire later on.
(A box with sand is a cheap fire extinguisher).
Don't use Lithium batteries when flying in areas with large amounts of dry vegetation, as a crash
may result in a serious forest fire.
A new sort of Lithium (Saphion) cells has now been introduced into the market.
These cells are claimed safe since they don't burst into flames when abused like
the traditional Li-Ion-Polymer do.
Their safety aspects result from the incorporation of phosphates as the cathode
material, which are stable in overcharge or short circuit conditions and also have
the ability to withstand high temperatures without decomposing.
When abuse occurs, phosphates are not prone to thermal runaway and don't burn.
These cells have a nominal voltage of 3.2V, can be discharged down to 2V and
charged to 4.2V.
The recommended discharge rate is 5 to 6C continuous for a long life or higher
discharge rates for a shorter life.
For further details check out the manufacturer Valence Technology Inc

The lead acid batteries have much lower
energy/weight ratio than all those previously
mentioned. Which means that the lead acid
batteries are heavier for the same capacity.
They are not suitable to be used airborne, but
since they are rather cheap, they are often used
on the flying fields as ground power supply for
engine starters and/or to charge the smaller ones.
There are various versions of lead acid batteries:
The Gel-Cell, the Absorbed Glass Mat (AGM) and the Wet Cell.
The Gel-Cell and the AGM batteries cost about twice as much as the Wet Cell.
However, they store very well and do not tend to sulfate or degrade as easily as
the Wet Cell.
Lead acid batteries get "sulfated" when the soft lead sulfate normally formed on the
positive and negative plates' surfaces re-crystallises into hard lead sulfate when
the batteries are left uncharged during long time. This reduces the battery's
capacity and ability to be recharged.
Adding Silica Gel to the sulphuric acid turns the electrolyte into a solid mass that
looks like jelly, hence the name Gel-Cell. This prevents acid spillage even when
the battery is broken.
The sulphuric acid in AGM batteries is absorbed into fine fibreglass mats, they have
the same advantage of the gelled batteries but can take more abuse.
Both the Gel-Cell and AGM are the safest lead acid batteries one can use.
However, Gel-Cell and some AGM batteries require a slower charging rate. These
batteries may be damaged if fast charged on a conventional car charger.
There are sealed (maintenance free) and serviceable non-sealed Wet Cell
batteries. Non-sealed batteries are recommended in hot climates since distilled
water can be added through the filler caps when the electrolyte evaporates due
to the high environment temperature.
The lead acid batteries have a self - discharge rate of about 1% to 25% a month.
They will discharge faster at higher temperature. For example, a battery stored at
35°C (95°F) will self-discharge twice as fast than one stored at 24°C (75°F).
Lead acid batteries left uncharged during long time will become fully discharged
and sulfated. The best way to prevent sulfation is by periodically recharging the
battery when it drops below 80% of its charge.
It is possible to determine a non-sealed battery's charge status by measuring the
concentration of the sulfuric acid of the battery electrolyte ("battery acid") with a
hydrometer.
These batteries are built with different characteristics depending on application.
For example, starting batteries (also called SLI - Starting, Lightning, Ignition) have
the ability to deliver large starting current during very short time (cranking amps).
They have many thin plates of Lead "sponge", which gives a large surface area.
Starting batteries are mainly intended to start engines when the batteries seldom
get deep discharged, because if they often get deep discharged the Lead sponge
falls faster to the bottom of the cells, significantly reducing their lifespan.
Another type are the deep cycle batteries, which may be discharged down to 20%
of the full charge, time after time, without reducing their lifespan as their plates are
much thicker, however, these batteries lack the ability to deliver large current during
short time compared with the starting batteries.
Deep cycle batteries are therefore used where current is needed during long time,
such as in forklifts, golf carts or solar electric backup power.
If a deep cycle battery is also going to be used as a starting battery, it should be
oversized about 20% in relation to the recommended starting battery's size in order
to provide the same cranking amps.
The lead acid batteries have normally 3 or 6 cells connected in series.
Each cell has a nominal voltage of 2V resulting in a nominal pack voltage of 6V
and 12V respectively.
They are usually charged with a constant voltage of 2.4 - 2.5V per cell having the
charging current limited to 1/10C. It is not recommended charging these batteries
with a charging current exceeding 1/3C.
A lead acid battery pack is considered fully charged when the charging current
falls below 10mA and/or the cell voltage reaches 2.4 - 2.5V.
Should a lead acid battery be continuously left on charge (when used as power
backup); the charging voltage should not exceed 2.25 - 2.30V per cell.
It is also advisable to charge these batteries in a well-ventilated area/room, since
it produces hydrogen-oxygen gases that can be explosive and also the electrolyte
contains sulfuric acid that can cause severe burns.
Lead acid batteries' lifespan is about 4 to 8 years depending on the treatment.









0 comments:
Post a Comment